See other cases
Fish-mouth papilla – main duct IPMN
A 70-year-old patient, former chronic ethanol consumer, non-smoker, with a medical history of chronic bronchitis and benign prostatic hyperplasia, presented with epigastric pain and steatorrhea, with a progressive onset over approximately 3 months. Recent medical history included an abdominal ultrasound that raised suspicion of a tumor located in the pancreatic head.
Clinical examination:
The abdomen was spontaneously tender and painful on deep palpation in the epigastric region, with no other significant clinical findings.
Laboratory investigations:
Mild normochromic, normocytic anemia (Hb = 11.3 g/dL). CA 19-9, CEA, and HbA1c were within normal limits.
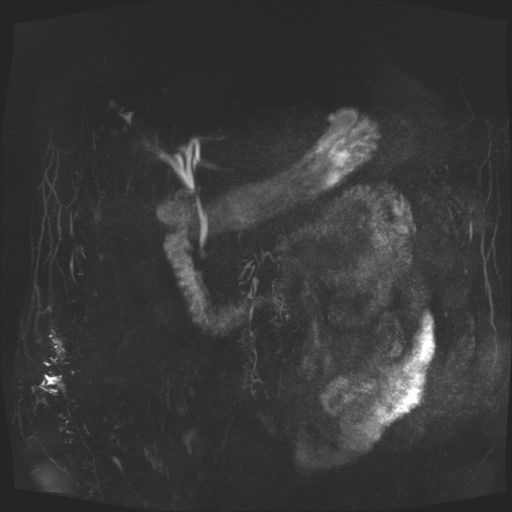
Magnetic Resonance Imaging (MRI)
Abdominal MRI revealed enlargement of the pancreatic head region due to a tumor with solid and cystic components, hyperintense on T2-weighted sequences and hypointense on T1-weighted images, with areas of diffusion restriction and heterogeneous gadolinium enhancement of the tissue components. The lesion measured approximately 65/50/60 mm.
The lesion lacked a clear cleavage plane from the first part of the duodenum and the posterior gastric wall in the antral-pyloric region. It was in contact with the main pancreatic duct, which was diffusely dilated along its entire course, predominantly in the body and head segments, with a maximum diameter of approximately 14 mm, containing dependent hypointense images suggestive of mucinous content.
Dilatation of secondary pancreatic ducts, the common bile duct, and intrahepatic bile ducts was also noted. No locoregional vascular invasion was identified; mesenteric vessels were patent, and the splenoportal axis was patent and non-dilated. Small, infracentimetric lymph nodes were observed in the celiac and lombo-aortic regions. No intraperitoneal free fluid was present.
Endoscopic findings
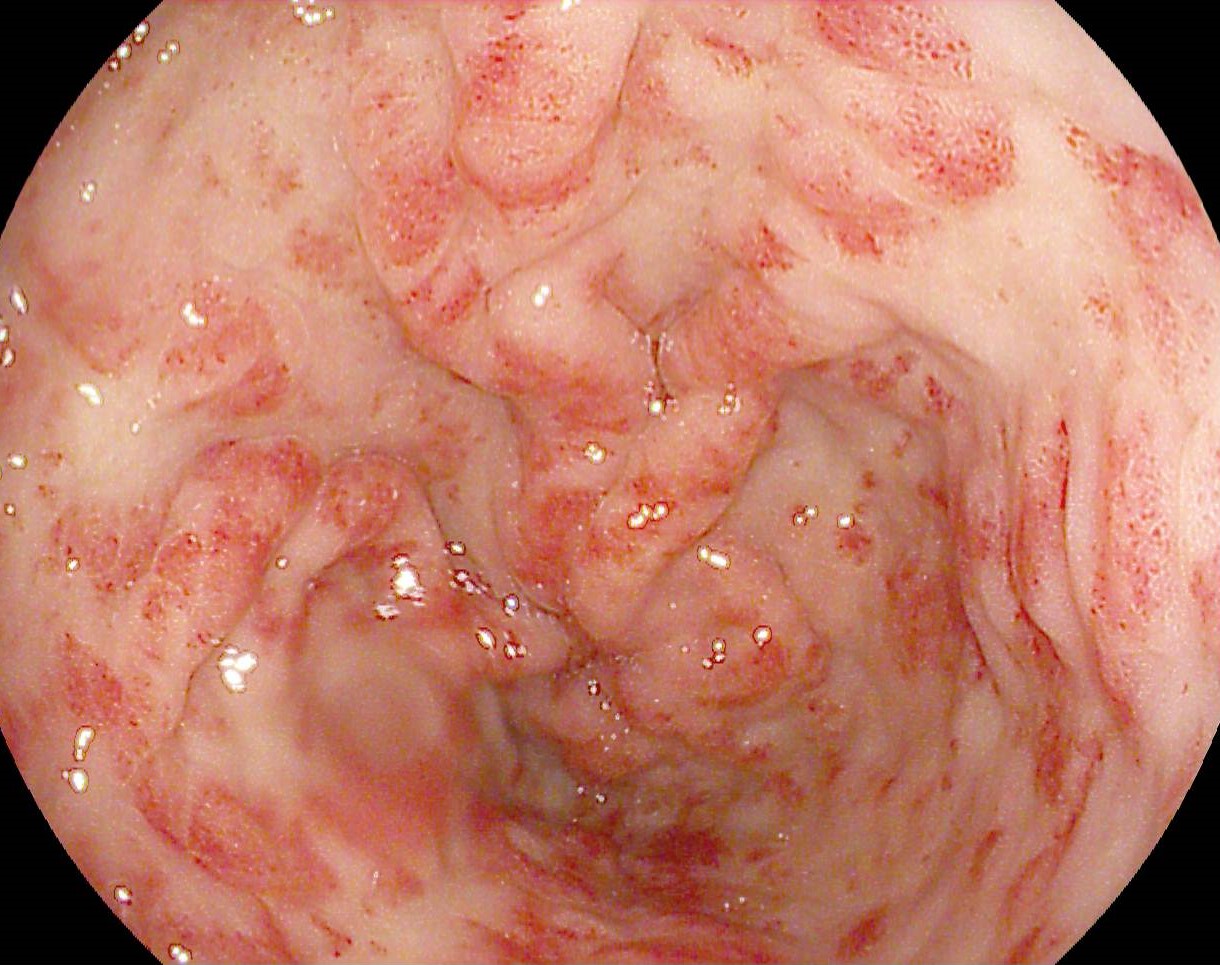
- Infiltrative appearance of the pyloro-bulbar mucosa, with difficulty in passing the echoendoscope at this level;
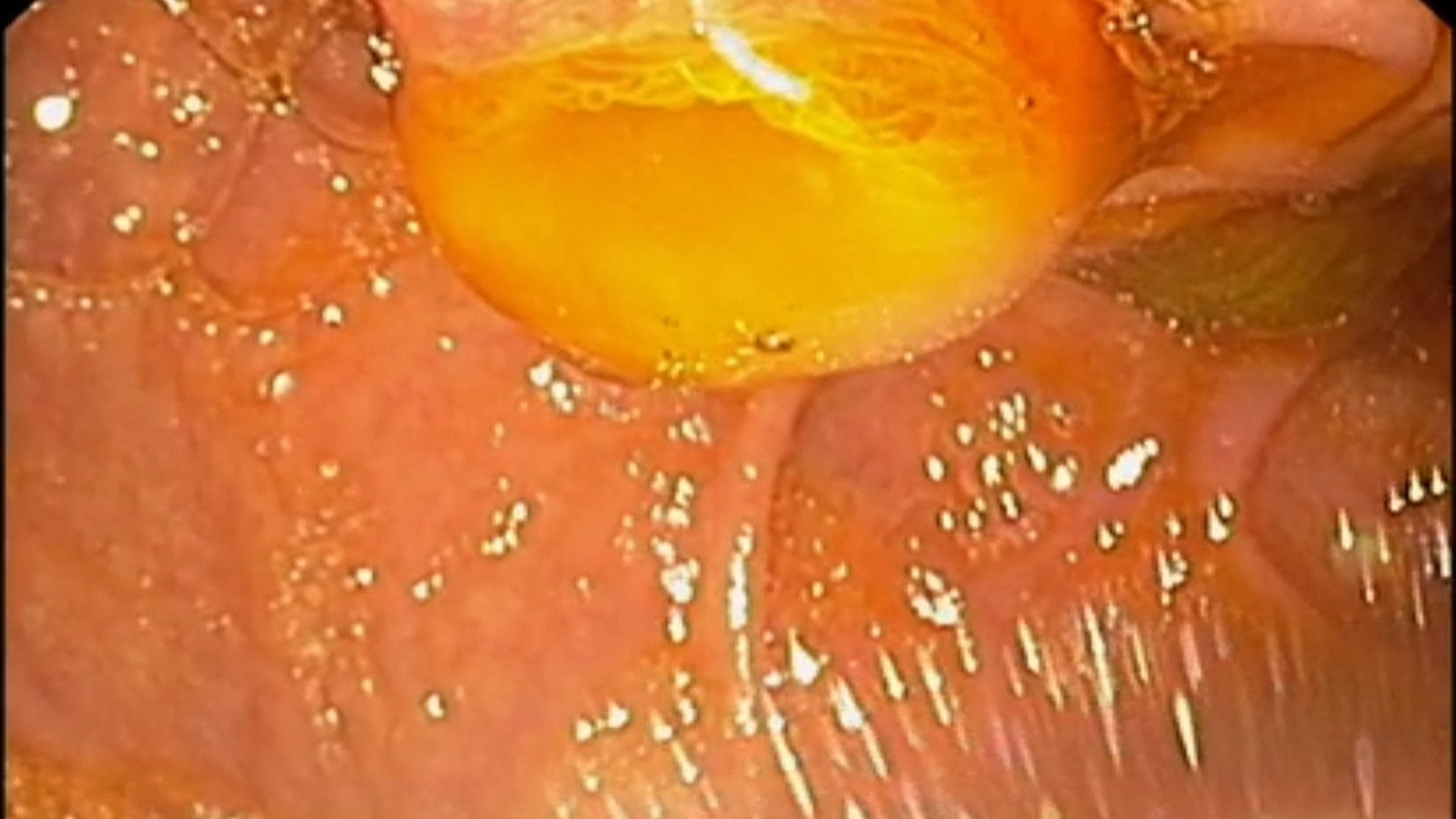
- “Fish-mouth” appearance of the duodenal papilla.
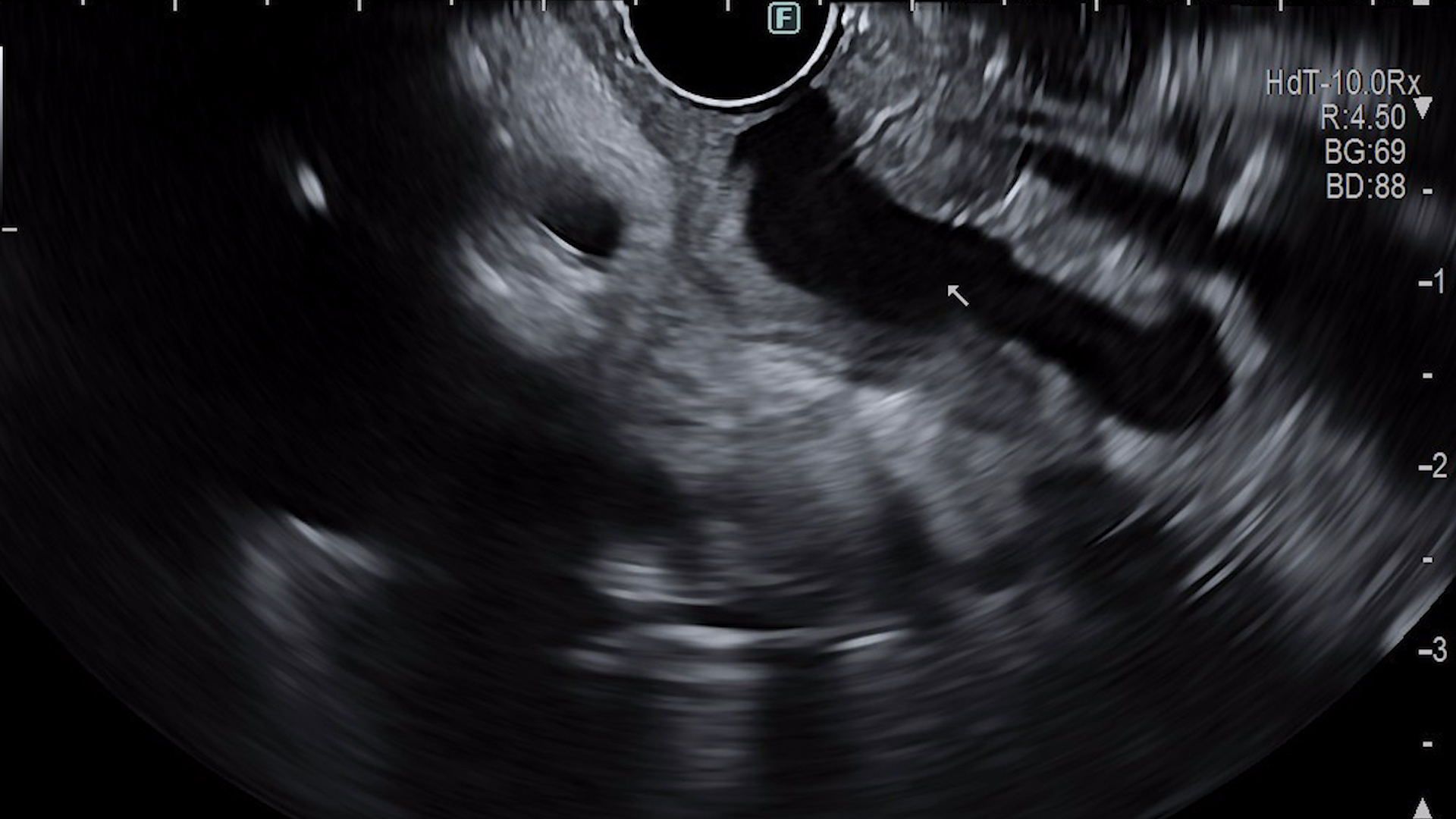
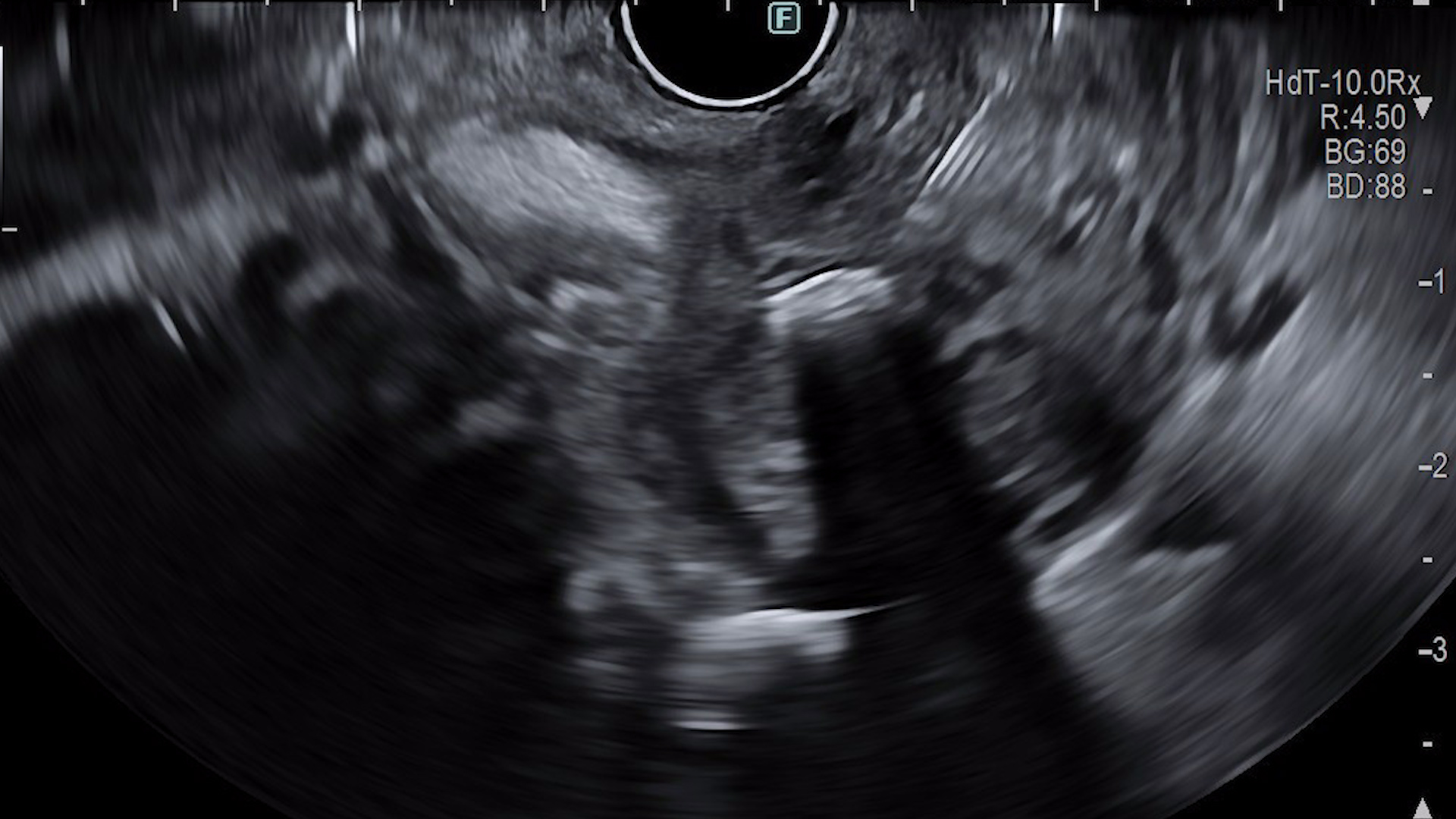
EUS evaluation
- Infiltrative changes of the pancreatic parenchyma with extension to the gastric and bulbar walls;
- Globally atrophic pancreatic parenchyma;
- Diffuse dilation of the main pancreatic duct, with a tortuous course, containing echogenic material suggestive of mucin, as well as hyperechoic images with posterior acoustic shadowing, mobile upon needle manipulation, suggestive of calcified mucin;
- Dilatation of secondary pancreatic ducts.
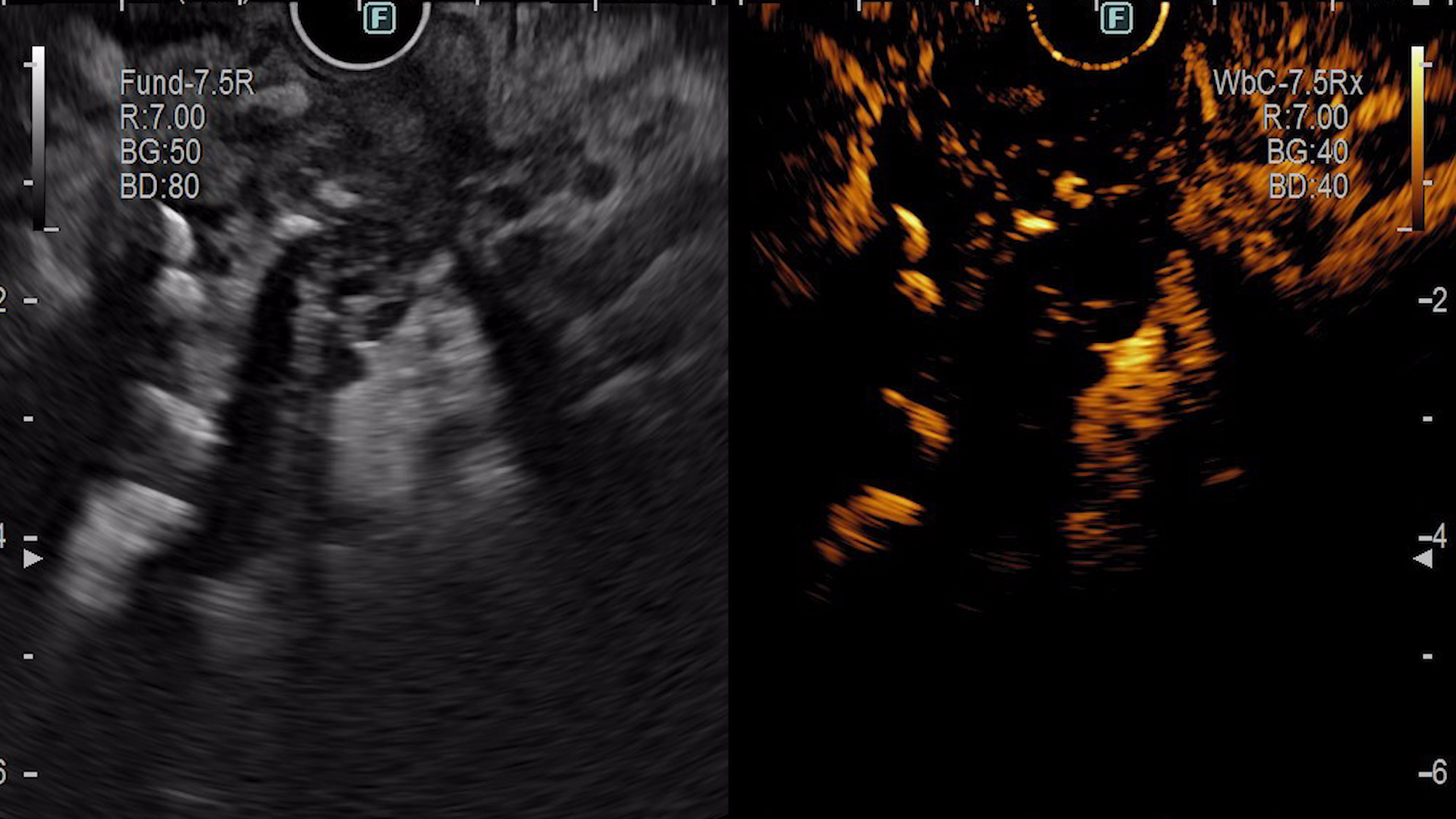
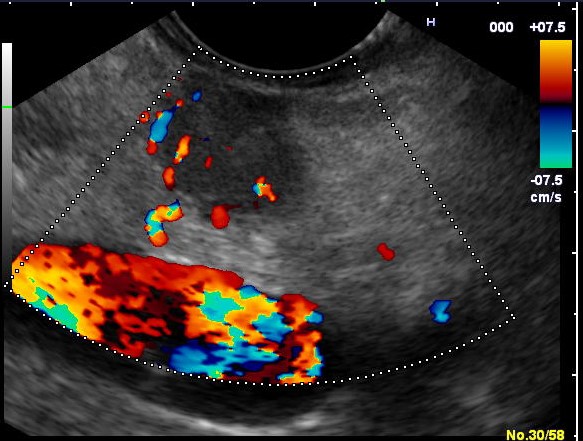
Contrast-enhanced EUS (CE-EUS)
After intravenous administration of 4.8 mL SonoVue, a poorly delineated, hypoenhancing area with heterogeneous echostructure was identified in the pancreatic head, measuring approximately 40/26 mm. The lesion was sampled by EUS-guided fine-needle biopsy (EUS-FNB), with two passes using a 22G needle (Acquire, Boston Scientific).
Histopathological Examination
Histopathological evaluation of the specimens obtained by EUS-guided biopsy revealed features suggestive of intraductal papillary mucinous neoplasm, with low-grade dysplasia. No high-grade dysplasia or definite signs of invasive neoplasia were identified in the examined material.
- Main-duct intraductal papillary mucinous neoplasm (MD-IPMN) with low-grade dysplasia;
- Chronic pancreatitis.
Intraductal papillary mucinous neoplasm (IPMN) is a distinct entity of pancreatic cystic neoplasms, characterized by excessive mucin production and pancreatic duct dilation, with a variable potential for malignant transformation. The main-duct subtype (MD-IPMN) is associated with the highest risk of progression to pancreatic adenocarcinoma. (1)
Pancreatic cystic lesions are increasingly detected due to the widespread use of imaging studies, with a higher prevalence on MRI (19.9%) compared with CT (2.6%). For cysts larger than 5 mm, detailed characterization with pancreatic-protocol CT or contrast-enhanced MRI with magnetic resonance cholangiopancreatography (MRCP) is recommended. MRCP is the diagnostic modality of choice for assessing ductal extension and relationships with adjacent structures, while also allowing identification of septa, mural nodules, and communication with pancreatic ducts. Additionally, MRI avoids radiation exposure during repeated follow-up examinations. (2)
EUS is recommended for further lesion characterization in patients presenting “worrisome features” on imaging, including cyst diameter ≥3 cm, enhancing mural nodules <5 mm, thickened and enhancing cyst walls, main pancreatic duct diameter of 5–9 mm, abrupt change in main pancreatic duct caliber with distal pancreatic atrophy, lymphadenopathy, elevated serum CA 19-9 levels, and rapid cyst growth (>5 mm/2 years). (2)
Although present in only approximately half of cases, the fish-mouth papilla appearance is considered pathognomonic for pancreatic IPMN and has a high specificity of 91%. (3)
In the present case, histopathological examination revealed low-grade dysplasia; however, lesion heterogeneity and reactive changes associated with chronic pancreatitis-related inflammation may lead to underestimation of the true risk of malignant transformation, thereby increasing the likelihood of a false-negative result. In this context, therapeutic decision-making should not rely exclusively on histological findings but rather on a comprehensive correlation of clinical, imaging, and endoscopic data. (4,5)
Given the high rate of malignancy associated with MD-IPMN, surgical resection remains the standard of care for main-duct lesions. In clinical practice, MD-IPMN with a duct diameter ≥10 mm represents an absolute indication for surgery, while lesions measuring between 5 and 9.9 mm constitute a relative indication that must be individually assessed. (4)
In conclusion, this case highlights the importance of a multimodal approach in the diagnosis of MD-IPMN and underscores the central role of EUS and MRI in identifying high-risk features with direct impact on therapeutic decision-making.
- Levink I, Bruno MJ, Cahen DL. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr Treat Options Gastroenterol. 2018 Sep;16(3):316–32.
- Tanaka M, Fernández-del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017 Sep;17(5):738–53.
- Asokkumar R, Chin YK. Fish-Mouth Papilla. Clin Gastroenterol Hepatol. 2018 Sep;16(9):A34.
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018 May;67(5):789–804.
- Serinelli S, Khurana KK. Intraductal papillary mucinous neoplasms of the pancreas: Cytologic-histologic correlation study and evaluation of the cytologic accuracy in identifying high-grade dysplasia/invasive adenocarcinoma. CytoJournal. 2024;21:6.