See other cases
EUS-guided pseudocyst drainage
A 45-year-old man with a history of mild acute pancreatitis presented six months after the initial episode for evaluation of a cephalic pancreatic pseudocyst. On CT, the pseudocyst measured 50 × 45 mm, had clear content, and thin walls.
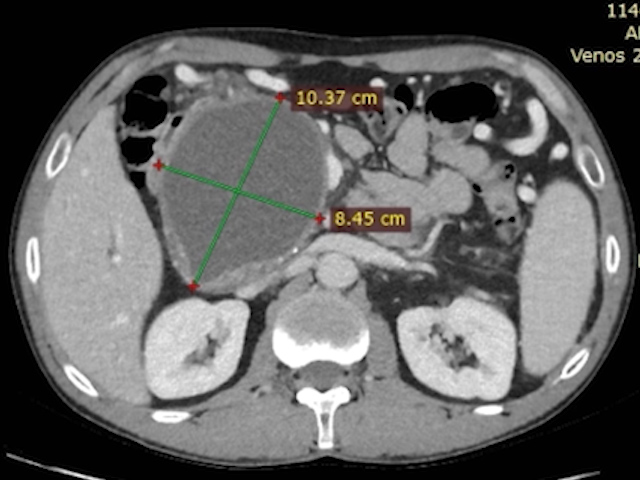
At admission, the pseudocyst had increased to 103 × 85 mm, causing compressive effects on the hepatic hilum (Fig. 1). The patient reported generalized pruritus and postprandial abdominal discomfort for approximately one month. On examination, he had scleral icterus, a soft but tender upper abdomen, and no signs of peritoneal irritation.
Laboratory tests revealed cholestasis (total bilirubin 3.4 mg/dL, direct bilirubin 2.5 mg/dL, GGT 300 IU/L, alkaline phosphatase 320 IU/L), while the complete blood count and inflammatory markers were within normal limits.
Linear-array endoscopic ultrasound (EUS) was performed to assess the feasibility of drainage and to exclude other causes of cholestasis.
The pancreas showed features of chronic pancreatitis, including a main pancreatic duct (MPD) dilated up to 7 mm with a beaded appearance and echogenic walls, dilated secondary ducts, and intraparenchymal calcifications. Discrete echogenic strands delineated hypoechoic lobules, and secondary ducts displayed segmental dilations.
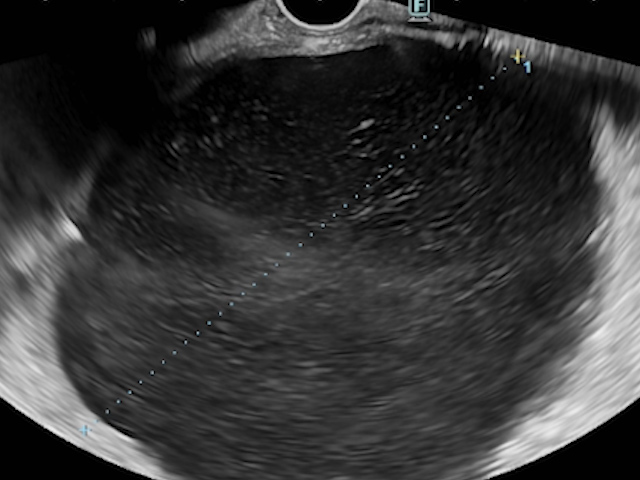
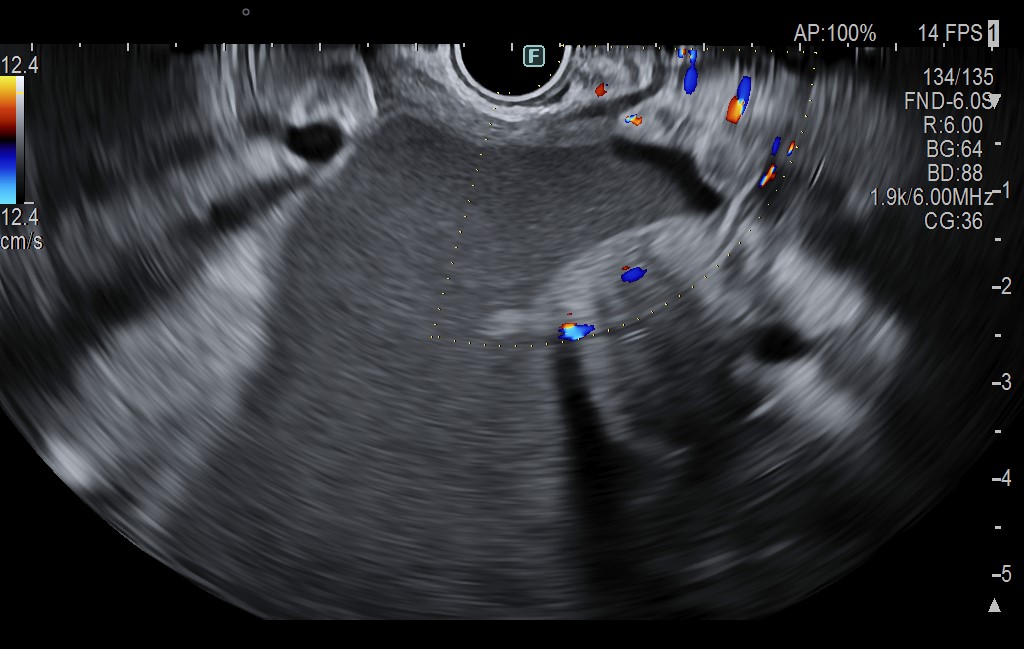
The cephalic pancreatic pseudocyst measured 83.5 mm, without solid components, and had a thin wall of 2.7 mm. Gastric station visualization was adequate, providing an optimal approach for EUS-guided cystogastrostomy (CGA-EUS) (Fig. 2).
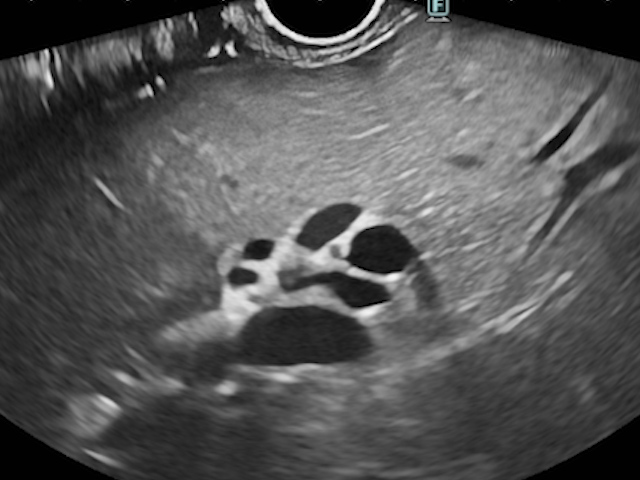
The echoendoscope was also positioned in the duodenal station to follow the MPD at the papilla, which appeared compressed but did not communicate with the fluid collection. The common bile duct (CBD) was dilated up to 14.6 mm, and intrahepatic bile ducts were dilated in both lobes (Fig. 3).
The diagnosis of calcific chronic pancreatitis with an obstructive cephalic pancreatic pseudocyst and cholestatic syndrome was established.
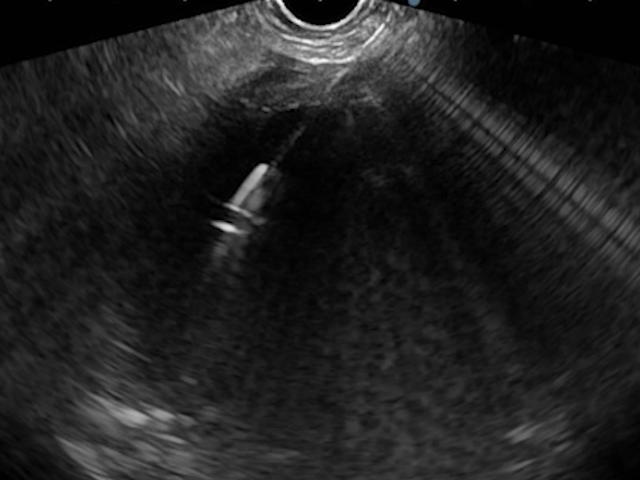
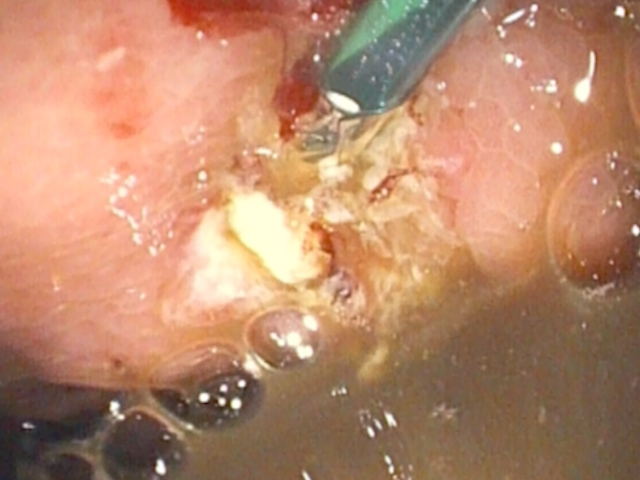
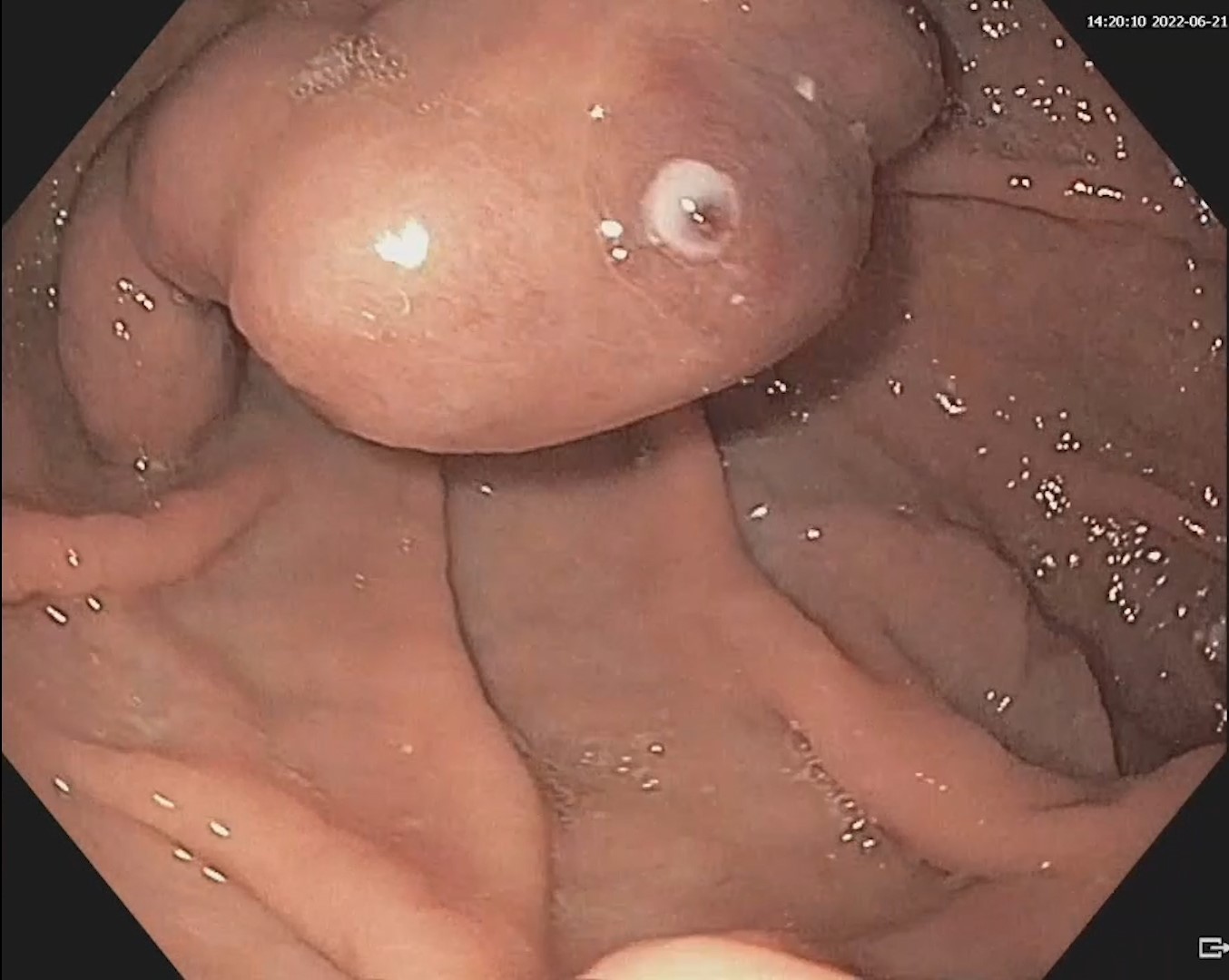
An EUS-guided CGA was performed using a plastic pigtail stent. Under deep sedation and endotracheal intubation, the collection was punctured with a 19G FNA needle, and the aspirate, with a brownish appearance typical of a pseudocyst, was sent for biochemical and microbiological analysis (Fig. 4).
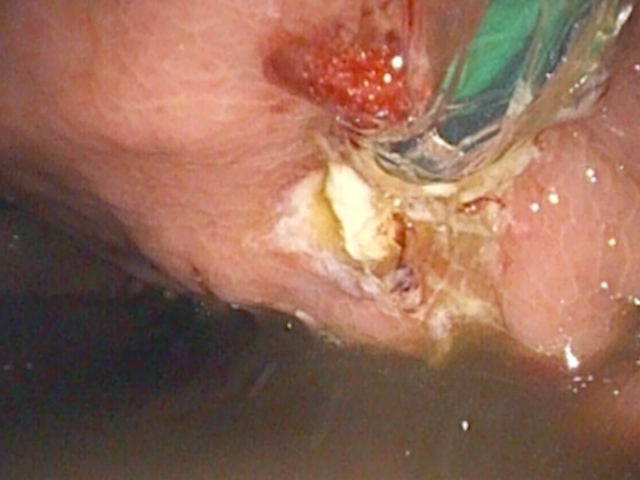
A 0.0035-inch guidewire was advanced and looped twice within the collection under fluoroscopic guidance. A 10G cystotome (Cook) was used to perform cystotomy, perforating both the gastric wall and the collection wall using Autocut 110 W (Fig. 5).
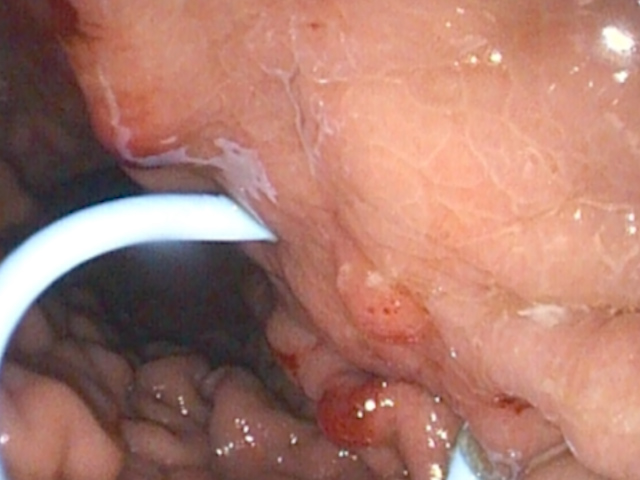
The tract was then dilated with an 8-mm balloon (Fig. 6), and the procedure was completed with the placement of a 10 Fr / 5 cm biliary pigtail stent (Fig. 7). The procedure was uneventful, and the patient returned to the gastroenterology ward 4 hours after extubation.
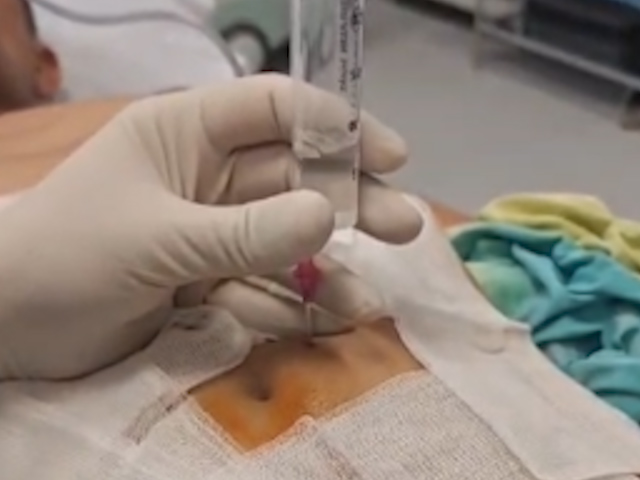
Within 24 hours, pruritus resolved, and cholestatic parameters normalized, although the patient experienced mild abdominal discomfort. A minor pneumoperitoneum was identified and managed conservatively with simple percutaneous drainage (Fig. 8). The patient was discharged asymptomatic 48 hours later. At the 30-day follow-up, the pseudocyst had significantly decreased to 27 mm, with the biliary stent in situ.
Local complications of acute pancreatitis occurring 4–6 weeks after the episode include necrotic collections or walled-off necrosis (WON) and pancreatic fluid collections (PFC) [1].
EUS-guided drainage is the preferred endoscopic treatment for PFC, replacing most surgical or radiologic approaches, with comparable clinical success but lower morbidity and cost [2]. Drainage can be achieved using plastic pigtail stents (SPP) or lumen-apposing metal stents (LAMS). LAMS are recommended for WON, whereas SPP are preferred for pseudocyst drainage [3–5]. Multiple stents or guidewires may be used simultaneously; the efficacy of a single SPP has been reported at 94% [6]. Multiple stents maintain the fistula, allow drainage through and between stents, and reduce the risk of migration, with reported success rates of 83–100% [7,8]. Smaller stents (e.g., 7 Fr) have higher obstruction rates; larger diameters are preferred, although the use of 7 Fr stents is not incorrect [2]. Fluoroscopy optimizes visualization, access, and device positioning but is not mandatory. A prospective study by Seicean et al. reported technical success of 83.3% (20/24) using SPP without fluoroscopy; all failures occurred in PFC <6 cm with wall thickness >2 mm [9].
The most common complications of EUS-guided cystogastrostomy include bleeding, infection, perforation, stent migration or obstruction, pneumoperitoneum, delayed pseudoaneurysmal bleeding, and persistent gastric fistula [10,11]. Pneumoperitoneum without peritonitis, a rare complication (<5%), typically results from transient air leakage into the peritoneum during tract dilation or minor air passage through the fistulous channel post-procedure. In the absence of peritoneal fluid or clinical signs of peritonitis, conservative observation and, if necessary, simple percutaneous drainage can be safely employed [12].
This case demonstrates the efficacy of EUS-guided drainage of a cephalic pancreatic pseudocyst with a plastic stent, the safety of conservative management of minor pneumoperitoneum, and the importance of stent selection based on the content of the collection, in accordance with ESGE guidelines.
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013 Jan;62(1):102-11. doi: 10.1136/gutjnl-2012-302779. Epub 2012 Oct 25. PMID: 23100216.
- Teoh AY, Dhir V, Jin ZD, Kida M, Seo DW, Ho KY. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc. 2016 Mar 25;8(6):310-8. doi: 10.4253/wjge.v8.i6.310. PMID: 27014427; PMCID: PMC4804189.
- Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Updated August 2018. Endoscopy. 2019 Feb;51(2):179-193. doi: 10.1055/a-0822-0832. Epub 2019 Jan 17. PMID: 30654394.
- van der Merwe SW, van Wanrooij RLJ, Bronswijk M, Everett S, Lakhtakia S, Rimbas M, Hucl T, Kunda R, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Perez-Miranda M, van Hooft JE. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391. Epub 2021 Dec 22. PMID: 34937098.
- van Wanrooij RLJ, Bronswijk M, Kunda R, Everett SM, Lakhtakia S, Rimbas M, Hucl T, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Pérez-Miranda M, van Hooft JE, van der Merwe SW. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022 Mar;54(3):310-332. doi: 10.1055/a-1738-6780. Epub 2022 Feb 3. PMID: 35114696.
- Lin H, Zhan XB, Sun SY, Yang XJ, Jin ZD, Zou DW, Li ZS. Stent selection for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a multicenter study in china. Gastroenterol Res Pract. 2014;2014:193562. doi: 10.1155/2014/193562. Epub 2014 Jun 11. PMID: 25018767; PMCID: PMC4074944.
- Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998 Aug;48(2):200-3. doi: 10.1016/s0016-5107(98)70165-8. PMID: 9717789.
- Seifert H, Dietrich C, Schmitt T, Caspary W, Wehrmann T. Endoscopic ultrasound-guided one-step transmural drainage of cystic abdominal lesions with a large-channel echo endoscope. Endoscopy. 2000 Mar;32(3):255-9. doi: 10.1055/s-2000-93. PMID: 10718392.
- Seicean A, Stan-Iuga R, Badea R, Tantau M, Mocan T, Seicean R, Iancu C, Pascu O. The safety of endoscopic ultrasonography-guided drainage of pancreatic fluid collections without fluoroscopic control: a single tertiary center experience. J Gastrointestin Liver Dis. 2011 Mar;20(1):39-45. PMID: 21451796.
- Puri R, Thandassery RB, Alfadda AA, Kaabi SA. Endoscopic ultrasound guided drainage of pancreatic fluid collections: Assessment of the procedure, technical details and review of the literature. World J Gastrointest Endosc. 2015 Apr 16;7(4):354-63. doi: 10.4253/wjge.v7.i4.354. PMID: 25901214; PMCID: PMC4400624.
- Kinoshita K, Okamoto K, Noguchi H, Fukuchi S, Akiyama H, Motomura M, Azuma Y, Hiroshima Y, Fuchino T, Ozaka S, Sagami R, Uchida T, Hirashita Y, Fukuda K, Ogawa R, Mizukami K, Kodama M, Murakami K. Efficacy of Endoscopic Ultrasound-guided Transluminal Drainage Using Lumen-apposing Metal Stents for the Treatment of Pancreatic Fluid Collections. DEN Open. 2025 Nov 22;6(1):e70249. doi: 10.1002/deo2.70249. PMID: 41287748; PMCID: PMC12640457.
- Prochazka Zárate R, Vidales Mostajo G, Villa-Gómez Roig G, Illescas Castellanos A, Pereira Robles N. Neumoperitoneo a tensión como complicación de drenaje transgástrico de pseudoquiste pancreático guiado por ultrasonografía endoscópica. Reporte de caso clínico y revisión de la literatura [Tension pneumoperitoneum as a complication of endoscopic ultrasound guided transgastric drainage of pancreatic pseudocyst: case report and review of the literature]. Rev Gastroenterol Peru. 2012 Jan-Mar;32(1):88-93. Spanish. PMID: 22476184.